ABOUT THE AUTHOR
The first author of this series of research is Dr. Xiaozhao Wang, postdoctoral fellow at ZJU-UoE Institute, and the co-first authors are Dr. Junxin Lin, postdoctoral fellow and Qin Wu, undergraduate at ZJU-UoE Institute,Professor Hongwei Ouyang is the correspondent of this paper. The research was supported by the National Natural Science Foundation of China Innovation Group Project T2121004 and so on. This research was also selected as one of the International Campus 2022 Academic Achievement Awards.
Dr. Xiaozhao Wang graduated from School of Materials Science and Engineering, Zhejiang University, and his postdoctoral research direction is high-definition analysis and precise regeneration of the complex structure of the human motion system.

INTRODUCTION
Professor Hongwei Ouyang's team from Zhejiang University has published a paper titled "Stage-specific and location-specific cartilage calcification in osteoarthritis development" in Annals of the Rheumatic Diseases. For the first time, they have explored the bidirectional pathological mechanisms of cartilage calcification in osteoarthritis (OA) development, known as "top-down" and "bottom-up," from the perspectives of molecular assembly, micro-nanostructure, and microscale mechanics. The results showed that OA progressed by bidirectional cartilage calcification from both top-down at the joint surface and bottom-up at the osteochondral interface at the very early stage of OA. The top-down calcification showed a gradual calcification process at the joint surface, and the bottom-up calcification formed a calcified sandwich structure first and fused in OA progression at calcified cartilage. Following their initial work on the high-resolution structures of osteochondral interface tissues of human knee joints (Nano Letters, 2022), this work refines our current understanding of the mechanism underlying OA progression and provides insights into the material science mechanisms of pathological calcification in OA development.
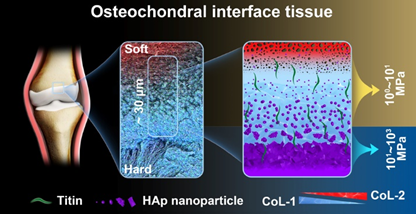
The osteochondral interface tissue in the human knee joint is characterized by a complex structure and experiences harsh mechanical environments. The normal osteochondral interface tissues maintain fatigue-resistant adhesion between the different materials of cartilage and bone. It also ensures effective force transmission over a lifetime of loading cycles. The interface’s secret to successfully preventing such damage should lie in its well-designed micro-nano structures and graded compositions in multiple levels. Thus, revealing this ultrathin interface is key to understanding its superb mechanics and for the future design of soft-hard composite interface materials. Against this backdrop, the team led by OUYANG Hongwei at the Zhejiang University conducted a high-resolution analysis of the osteochondral interface in human knee joints. By employing various high-resolution characterization techniques, they discovered the presence of a unique ultrathin 20~30 μm graded calcified region at the osteochondral interface (Fig 1). The characteristic biomolecular compositions, well-organized mineral assemblies with nanoscale heterogeneity, and exponential increment of tissue stiffness together contribute to its exceptional mechanical properties (Nano Lett. 2022, 22, 2309-2319).
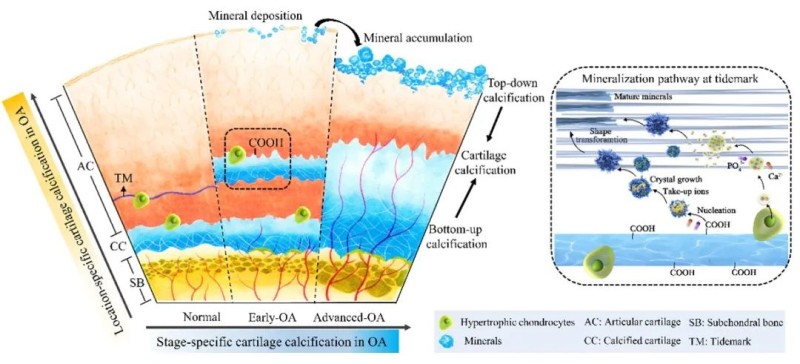
Alterations in the ultrastructural assembly of the osteochondral interface can easily induce osteoarthritis (OA). Currently, cartilage degradation and subchondral bone remodeling have been extensively studied and are considered synchronous phenomena in OA. However, little is known about the pathological changes in the ultrastructure, molecular assembly, and mechanics of their interface tissue, “osteochondral interface”, during the development of OA. Therefore, the research team investigated the process and mechanisms of the “bottom-up calcification” from osteochondral interface at different stages of OA (Fig 2). The study revealed that in the early stage of OA, a characteristic " bottom-up calcification" emerged, starting from the calcified cartilage, and forming a sandwich-like calcified structure. Acidic substances near the tidemark and hypertrophic chondrocytes promoted the formation of this characteristic structure. As OA progressed, the calcified sandwich structure fused, leading to calcified cartilage region thickening. During early stage of OA, the calcified cartilage was hypermineralised, containing stiffer carbonated hydroxyapatite (HAp). During advanced stage of OA, it was hypomineralised and contained softer HAp. This discrepancy may be attributed to matrix vesicle nucleation during OA-E and carbonate cores during advanced stage of OA.
AUTHOR'S NOTE
This study involves multiple cross fields such as medicine, life sciences, materials science, and mechanics. We integrated multi-scale and multi-modal methods to explore the 20-30μm structure and component assembly mechanism of human knee joint osteochondral interface. We explored a lot of tissue analysis and nano-scale characterization methods from the synchrotron radiation station of Shanghai Advanced Research Institute, Chinese Academy of Sciences, frozen electron microscope of Tsinghua University, mass spectrometry platform of Westlake University to multiple characterization platforms of Zhejiang University, and worked with experts in different areas. The research has gone through more than two years of review-revision stage, as well as repeated processes of major revision-minor revision - rejection, and was finally published in Nano Letters on March 3, 2022.
Furthermore, we analyzed the osteochondral interface of OA pathology in an attempt to clarify the influence of the change of osteochondral interface on the development of OA pathology from the perspective of structure, composition, and mechanics. The test results are really interesting. We found that in the early and late OA, the mineral assembly and mechanics at the osteochondral interface showed a completely opposite trend. Further analysis revealed that the root of the difference lies in the different paths of HAp synthesis and assembly and the different nucleation mechanisms regulated by hypertrophic chondrocytes. In addition to the osteochondral interface, we unexpectedly found a pearl-like nano HAp sphere on the upper layer of cartilage in the early stage of OA. With the development of OA, the sphere gradually transformed into a polygonal and dense assembly state, and gradually invaded the lower layer of cartilage, showing the overall invasion trend of bottom-up and top-down pathological calcification from the cartilage surface and osteochondral interface. In order to complete this study, we collected more than 50 human joint replacement samples repeatedly, and conducted histological and electron microscopic observations on each sample, and collected more than 5000 electron microscope images, and statistically observed the structural transformation process of OA pathological cartilage. Meanwhile, we cooperated with Professor Huan Hu of ZJUI to characterize the nano-mechanical properties of various samples by using their atomic force microscope (AFM). Combining theses properties closely with OA pathological calcification process and OA progress, the mechanism of the occurrence and development of pathological calcification in OA pathology was deeply explored and discussed. The study was published on October 19, 2022 in Annals of the Rheumatic Diseases.
Experience: 1. Scientific research is full of uncertainties, and we are not sure the results before the test; however, t it's the unknown results that are really worth exploring; 2. Scientific research is also biased, but research that gradually makes breakthroughs and perfects itself will certainly be appreciated and respected.
Academic Achievement Award

International Campus selects the most commendable academic achievements of the year by the end of each year. In 2022, a total of 10 research achievements including this series of research were selected, which will be introduced in the Science 101 column.





